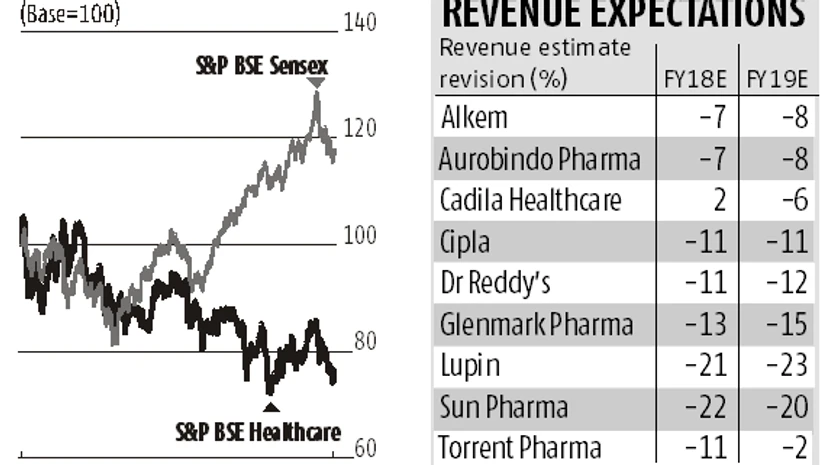
If FY18 was a forgettable year for the pharma sector, FY19 might not be any better. This is on account of improved pace of approvals from April 1, 2018 as well as competition even for drugs, which are difficult to make. New guidelines from the US Food and Drug Administration had led to the slowing down in drug approvals over the last couple of months as companies took time to adjust. However, as companies comply with the new guidelines, the approval time for drugs from April is expected to come down further increasing competitive pressures. Anubhav Aggarwal and Chunky Shah
More pain ahead for drug makers in FY19 after a challenging FY18
Higher number of approvals from April 2018, repeated regulatory issues and lower profits from niche opportunities are some of the concerns
)
Premium
What you get on BS Premium?
-
Unlock 30+ premium stories daily hand-picked by our editors, across devices on browser and app.
-
Pick your 5 favourite companies, get a daily email with all news updates on them.
Full access to our intuitive epaper - clip, save, share articles from any device; newspaper archives from 2006.
Preferential invites to Business Standard events.
Curated newsletters on markets, personal finance, policy & politics, start-ups, technology, and more.
Need More Information - write to us at assist@bsmail.in
