Ahmedabad-headquartered pharma major Zydus Cadila has got the final nod from the US drug regulator to market Mesalamine tablets in the US. The ulcerative colitis drug is estimated to have a market size of $1.145 billion.
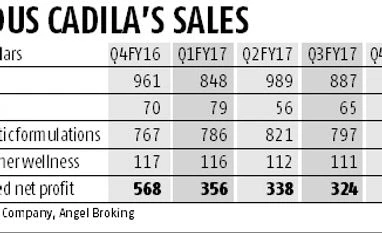
While most pharma majors are witnessing degrowth in the US, Zydus Cadila (or Cadila Healthcare) has managed to post a 2.5 per cent rise in its US revenues to Rs 985 crore during the fourth quarter of FY17. Analysts feel the launch of this blockbuster drug that has limited competition is will further boost its US business in FY18. Angel Broking estimated the company can add $60 million in sales and $20 million in net profit in the US market during the current financial year. Cadila now has nearly 115 approvals from the USFDA.
In fact, Zydus was the first to file an abbreviated new drug application (ANDA) for a generic version of Lialda (Mesalamine) in the US. In May, Zydus had said it had received a favourable judgment from a US federal court in a patent infringement case in favour of its US subsidiary, Zydus Pharmaceuticals (USA) Inc. The United States Court of Appeals for the Federal Circuit has affirmed the judgment in favour of its US subsidiary, Zydus Pharmaceuticals, holding that its proposed generic version of Lialda (Mesalamine) does not infringe US Patent No. 6,773,720. This basically paved the way for the launch of generic Lialda in US.
Irish pharma company Shire's Lialda treats ulcerative colitis, a form of irritable bowel disease that affects approximately 700,000 people in the US. In February, Shire had announced total sales of $792 million for Lialda in 2016, which had grown 16 per cent over last year.
The generic version of Lialda, thus, presents a significant opportunity for Zydus in the US.
The US market contributes around 40 per cent to Zydus Cadila’s sales. According to Angel Broking, its overall revenue from the US market in FY17 was Rs 3,709 crore, or 40.22 per cent of its consolidated annual turnover of Rs 9,220 crore.
The sales growth in the fourth quarter for the US market was marginal at 2.5 per cent on a year-on-year basis but it was up 11 per cent on a quarter-on-quarter basis. In the third quarter, it had posted Rs 887 crore sales in the US, down from Rs 989 crore in the second quarter of the financial year.
Angel Broking expects overall exports, including to the US, Europe and emerging markets, to post a compounded annual growth of 23.7 per cent over FY17-19. New product launches are likely to play the key role.
The generic version of Lialda (Mesalamine) delayed release tablets 1.2gm would be produced at the company's Moraiya plant near Ahmedabad, which has recently received the USFDA approval for an antibacterial injection. Around 40 product approvals are expected from Moraiya, which contributes around 60 per cent to Zydus’ US sales.
The plant was served a warning letter in December 2015 by the USFDA, but is now out of regulatory trouble after the company successfully cleared an USFDA audit in February, with no observations.
The group now has more than 115 approvals and has so far filed over 300 ANDAs since the commencement of the filing process in 2003-04.
Cadila Healthcare shares on Wednesday closed higher 46.85 points, 9.55 per cent, at 537.25 on the BSE. Its market capitalisation crossed Rs 55,000 crore, making it the second most valuable pharma company in India.
Unlock 30+ premium stories daily hand-picked by our editors, across devices on browser and app.
Pick your 5 favourite companies, get a daily email with all news updates on them.
Full access to our intuitive epaper - clip, save, share articles from any device; newspaper archives from 2006.
Preferential invites to Business Standard events.
Curated newsletters on markets, personal finance, policy & politics, start-ups, technology, and more.
)