Russia is accusing the West of maligning its achievements in the global race to defeat Covid-19 as its attempts to win key markets for its Sputnik V vaccine run up against the demands of regulators.
“We understand the game,” Kirill Dmitriev, chief executive officer of the Russian Direct Investment Fund, which backed Sputnik V’s development and negotiates its international roll-out, said in an interview. “It’s a combination of some misunderstanding, some strong bias and, really, some very strong efforts to undermine the Russian vaccine.”
Like neighboring China, which is struggling to reassure nations testing its vaccines, Russia’s drive to convert what it calls a scientific triumph into geopolitical dividends has hit unexpected headwinds.
President Vladimir Putin has pushed the inoculation in calls with other world leaders since touting Russia’s approval of Sputnik V in August as the globe’s first Covid-19 vaccine. But many countries’ regulators have been unwilling to give Sputnik V fast-track approval -- even as they welcome U.S. and European vaccines that first completed comprehensive trials.
Kirill Dmitriev
The contest for access carries echoes of the Cold War space race triggered by the Soviet Union’s 1957 launch of the world’s first satellite, Sputnik, for which Russia’s vaccine is named. While Moscow was first into space, it was overtaken by the U.S. which landed a man on the moon 12 years later.
Russian officials blame Sputnik’s difficulties on bias. The Foreign Ministry recently described the vaccine race as the latest phase in a long-running disinformation “war” against Russia.
Regulators that have demanded more data say they’re just trying to ensure Sputnik V, which Russia approved weeks before Phase 3 studies to show its safety and effectiveness started, is as good as its backers say.
Take-up has been slow. It wasn’t until Dec. 21 that neighboring Belarus became the first country outside Russia to approve Sputnik V, and Argentina followed two days later. Argentina began vaccinations Tuesday with some 300,000 people expected to be given the Russian shot initially, and Belarus started its program the same day.
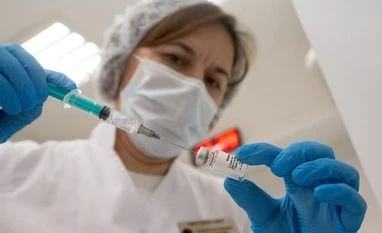
A health worker injects the Sputnik V vaccine into a patient's arm in Moscow, on Dec. 5.
A staff member receives the first dose of Sputnik V vaccine at Isidoro Iriarte Hospital in Quilmes, Argentina, on Dec. 29.
But India, Brazil and other major markets aren’t expected to sign off until next year, after more trials are done.
“Russia is using its vaccine program for soft power diplomacy,” said John Moore, a vaccine researcher at Weill Cornell Medical College in New York. “It’s an international race, there’s nationalism at stake. But it all depends on the vaccines being effective and safe.”
The Russian vaccine got a boost earlier in December when AstraZeneca PLC agreed to test a combination of its inoculation with one of the two shots that make up Sputnik V. Putin took part in the video-conference announcing the signing of the deal live on national television.
Still, the 68-year-old leader said Dec. 17 he was waiting to get the vaccine until it had been cleared for people his age.
Putin’s comments flummoxed Argentine officials, who had planned to start their campaign on the elderly. His spokesman this week indicated the president was now willing to get inoculated after research extended the age range for safe use of Sputnik V.
Critics say Russia’s decision to approve the vaccine so quickly, before its developers had published scientific data and after only limited trials, undermined confidence. Western officials including U.S. Secretary of State Mike Pompeo have described that move as premature, publicly questioning Sputnik’s safety.
A staff member receives the first dose of Sputnik V vaccine at Isidoro Iriarte Hospital in Quilmes, Argentina, on Dec. 29.
Russian officials brush those attacks off as unfair competition even as polls show many of Russia’s own citizens are skeptical about the safety of available vaccines.
While Dmitriev was upbeat in a September interview with India TV, his hopes of an immediate warm reception from regulators in other countries didn’t materialize.
‘Absolutely Confident’
“We are absolutely confident that it will receive emergency approval in a number of markets around the world already in November,” he said, arguing that Sputnik is “better, much safer” than western vaccines that use different technologies.
Sputnik V uses a platform based on the adenovirus, which causes the common cold, and has been studied in vaccine development for decades, though its effectiveness is yet to be proven. AstroZeneca’s is similar, while drugs developed by Moderna and Pfizer and BioNTech rely on a new technology, which uses genetic instructions in a nucleic acid molecule called mRNA to trick the body into making the viral protein itself, triggering an immune response.
Russia Begins Mass Covid-19 Vaccination Program
Russian officials play down the reverses, saying they’ve already got orders for 1.2 billion doses and plans to produce 500 million next year in several countries, while predicting other vaccine makers may struggle to meet the expected demand.
“We’re focused on regulators in Asia, the Mideast, Africa and Latin America, where political sentiment is more balanced,” Dmitriev said. He added that several are expected to follow Argentina and approve Sputnik V based on the Russian trials in January and February, with Venezuela the first in line. “People will understand there’s a major shortage of vaccine in 2021 and maybe 2022,” he said.
In India, hopes of fast-track regulatory approval for Sputnik V were dashed in October after authorities demanded more comprehensive trials than its local partners had proposed. RDIF said it hopes to apply for emergency-use approval by the end of January, but its Indian partner said the go-ahead isn’t likely until the second quarter of 2021.
It’s a similar story in Brazil, where Russia’s plan to start supplies in November failed to materialize. The Anvisa regulator said Tuesday it had received an application for Phase 3 trials of Sputnik V.
A month after the announcement of a production and distribution deal with a company in Beijing, Dmitriev said RDIF won’t sell Sputnik V inside China but instead export the millions of doses it plans to make there, including to Russia.
European Union member state Hungary has received 6,000 doses, though its regulator hasn’t yet cleared the drug for use.
While Russia may have hurt its credibility by rushing ahead, it will find markets for Sputnik V if it “can show the vaccine works and is reliable,” said Anthony McDonnell, a former U.K. government health adviser who’s now a senior policy analyst at the Washington-based Center for Global Development.
Unlock 30+ premium stories daily hand-picked by our editors, across devices on browser and app.
Pick your 5 favourite companies, get a daily email with all news updates on them.
Full access to our intuitive epaper - clip, save, share articles from any device; newspaper archives from 2006.
Preferential invites to Business Standard events.
Curated newsletters on markets, personal finance, policy & politics, start-ups, technology, and more.
)