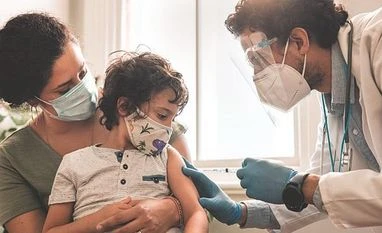
The US Food and Drug Administrationmay authorise the use of the Pfizer and BioNTech vaccine in the age group even though it did not meet a key target in a clinical trial of two- to four-year-olds. The drugmakers said they submitted data supporting authorisation at the request of the FDA in order to address an urgent public health need in the age group.
Outside advisors to the FDA are scheduled to meet on February 15 to discuss whether to recommend the regulator to authorise the vaccine. The roll out of the vaccine for children under the age of 5, the only age group not yet eligible for the shots, is set to begin less than a week after the meeting.
According to the CDC document, the government is planning to ship an initial 10 million doses of the Pfizer/BioNTech vaccine to states and other entities before the end of February, should the FDA authorise their use.
To read the full story, Subscribe Now at just Rs 249 a month
Already a subscriber? Log in
Subscribe To BS Premium
₹249
Renews automatically
₹1699₹1999
Opt for auto renewal and save Rs. 300 Renews automatically
₹1999
What you get on BS Premium?
-
Unlock 30+ premium stories daily hand-picked by our editors, across devices on browser and app.
-
Pick your 5 favourite companies, get a daily email with all news updates on them.
Full access to our intuitive epaper - clip, save, share articles from any device; newspaper archives from 2006.
Preferential invites to Business Standard events.
Curated newsletters on markets, personal finance, policy & politics, start-ups, technology, and more.
Need More Information - write to us at assist@bsmail.in
)