Promius Pharma Llc, a subsidiary of Dr Reddy’s Laboratories, has launched Zembrace SymTouch (sumatriptan injection), used for the treatment of migraine, in the US. In January this year, Dr Reddy’s received the US Food & Drug Administration’s approval for Zembrace SymTouch 3 mg for the acute treatment of migraine with or without aura in adults.
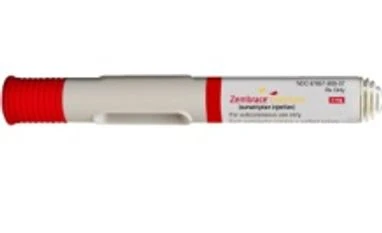
Zembrace SymTouch is a prefilled, low-dose, ready-to-use, 2-step autoinjector containing 3 mg of sumatriptan, a selective 5-HT1B/ID receptor agonist. Because Zembrace SymTouch is a subcutaneous injection, it may lead to rapid relief of migraine.
Migraineurs often have more than one kind of attack. Different types of medications can be used to treat different types of attacks. “Zembrace SymTouch is a welcome addition to the toolkit for acute treatment of migraine. This 3-mg, easy to-use subcutaneous injection of sumatriptan may be desired by many migraine patients who need a medication that can rapidly treat migraine attacks. Also, the 3-mg dose allows for up to 4 doses of Zembrace SymTouch in a 24-hour period, thus allowing dosing flexibility for patients,” said Roger Cady, associate executive chairman of the National Headache Foundation.
Zembrace SymTouch is available as a box of 4 autoinjectors.
Zembrace SymTouch is a prefilled, low-dose, ready-to-use, 2-step autoinjector containing 3 mg of sumatriptan, a selective 5-HT1B/ID receptor agonist. Because Zembrace SymTouch is a subcutaneous injection, it may lead to rapid relief of migraine.
Migraineurs often have more than one kind of attack. Different types of medications can be used to treat different types of attacks. “Zembrace SymTouch is a welcome addition to the toolkit for acute treatment of migraine. This 3-mg, easy to-use subcutaneous injection of sumatriptan may be desired by many migraine patients who need a medication that can rapidly treat migraine attacks. Also, the 3-mg dose allows for up to 4 doses of Zembrace SymTouch in a 24-hour period, thus allowing dosing flexibility for patients,” said Roger Cady, associate executive chairman of the National Headache Foundation.
Zembrace SymTouch is available as a box of 4 autoinjectors.
)