Off patent generic drugs manufactured in India are the key sources of cure for the diseases affecting the populations of the developed world. However, it needs to be seen if the western world is getting enough of those medicines for curing those diseases at the appropriate time intervals. The off patent drugs which any pharmaceutical company, including Indian, file at the USFDA are called as Abbreviated New Drug Approvals (ANDA).
Data collection and research findings from the US FDA website reveal that there has been a consolidation in the number of ANDA filings in the 4 year period of 2012-2015. The number of ANDA filings has practically remained steady despite the approval timelines for a generic drug application going to as high as 27-30 months. More than 2000 ANDA applications are approved each year and least 500 fall under the new drug approvals category each year considering the period from 2012-2015.
There is also another category of ANDA approvals and that is known as the tentative approval category. Many Indian pharmaceutical companies have received tentative ANDA approvals in the period 2012-2015. At least 100 drug applications fall under the tentative approval category each year and 50 percent of them are granted by the USFDA to Indian pharmaceuticals companies. Of the top 20 companies in the ANDA tentative approval list, 14 companies are Indian pharmaceutical manufacturers too.
Challenges to ANDA filings: The long wait
The filings which are done by the Indian pharmaceutical companies do not get the attention from the regulators at an accelerated pace. All relevant documents are filed with respect to the requirements of the ANDA filing along with fee for the filing. Once the filing has been received, the US FDA inspectors issue a letter for the manufacturing facility inspection at the place where the drug has been manufactured.
The time lag between the filing process and inspection is a fairly large and the inspection may take a week to 10 days or more based on the nature of the drug manufacturing process, facility and related parameters. Post inspection, the inspectors release their reports. If the report carries no queries, the approval process takes its route. If report carries findings, then a Form 483 is issued post inspection in which the manufacturer has to make amends to the manufacturing process at the facility and further re apply for ANDA approval or else in drastic circumstances withdraw the product completely. This entire process of approval takes around 25-30 months.
Challenges: Short supply drugs
Most of the medicines which are in short supply are injectables formulations particularly antibiotics (mainly cephalosporins). The results are then compiled to ascertain the possible potential of Indian drug exports of these products. Research shows that 19 key drug products are exported from India where USA is the largest consumer. Needs to be seen that for remaining drugs in the total of 62 short supply drugs, there stands a tremendous potential for the Indian pharmaceutical companies to enhance their export potential.
The future
The Indian pharmaceutical industry has dual avenues to enhance its export potential. One is the path of the regular ANDA approvals which despite being a slow and lengthy processes at the US FDA continues to remain the sunrise for the Indian pharmaceutical industry.
The second path is the short supply drug market which is one of the most important path for the growth of the Indian pharmaceutical exports. The US FDA website lists around more than 100 drugs, mainly injectables, which are in short supply. The issue of short supply for around 62 medicines is yet to be resolved by the USFDA. Of those 48 medicines are manufactured in India. But only around 19-20 medicines of those manufactured in India are exclusively shipped to USA.
Majority of the demand lies with the US market which accounts for more than 50 percent of the total export value in the short supply drug market. Despite the fact that more than 50 percent of the key shipments at the key ports are for the US consumption, there is still a short supply of drugs. This indicates that there exists a huge potential to simultaneously manufacture and export more drugs which are in the short supply in the US market.
___________________________________________________________________________________________________
Rashmi Pant is an expert in market research with more than 15 years of experience in major industrial sectors. She is also the owner of HOW TO: http://www.rashmipant.com
Data collection and research findings from the US FDA website reveal that there has been a consolidation in the number of ANDA filings in the 4 year period of 2012-2015. The number of ANDA filings has practically remained steady despite the approval timelines for a generic drug application going to as high as 27-30 months. More than 2000 ANDA applications are approved each year and least 500 fall under the new drug approvals category each year considering the period from 2012-2015.
There is also another category of ANDA approvals and that is known as the tentative approval category. Many Indian pharmaceutical companies have received tentative ANDA approvals in the period 2012-2015. At least 100 drug applications fall under the tentative approval category each year and 50 percent of them are granted by the USFDA to Indian pharmaceuticals companies. Of the top 20 companies in the ANDA tentative approval list, 14 companies are Indian pharmaceutical manufacturers too.
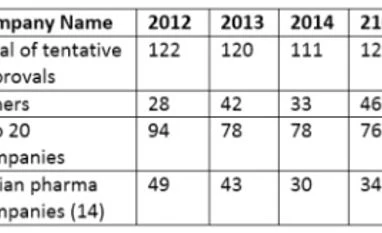
Tentative ANDA approval list of US FDA
For the top 20 companies, total tentative approvals were the highest in 2012 and are maintaining an average of 75 each year for the period post 2012 till date. Of the 14 Indian pharmaceutical companies (which are a part of the top 20 companies in tentative ANDA approvals), the contribution to the total tentative approvals was nearly 40 percent which has gradually reduced to 36 percent in 2013, 27 percent in 2014 and risen to nearly 28 percent in the 11 months of 2015 (34 approvals in 2015). If this trend continues, Indian pharmaceutical manufacturers will dominate the tentative ANDA approval list.Challenges to ANDA filings: The long wait
The filings which are done by the Indian pharmaceutical companies do not get the attention from the regulators at an accelerated pace. All relevant documents are filed with respect to the requirements of the ANDA filing along with fee for the filing. Once the filing has been received, the US FDA inspectors issue a letter for the manufacturing facility inspection at the place where the drug has been manufactured.
The time lag between the filing process and inspection is a fairly large and the inspection may take a week to 10 days or more based on the nature of the drug manufacturing process, facility and related parameters. Post inspection, the inspectors release their reports. If the report carries no queries, the approval process takes its route. If report carries findings, then a Form 483 is issued post inspection in which the manufacturer has to make amends to the manufacturing process at the facility and further re apply for ANDA approval or else in drastic circumstances withdraw the product completely. This entire process of approval takes around 25-30 months.
Challenges: Short supply drugs
Rashmi Pant
US FDA website has a listing for short supply drugs in their country. Out of the 110 drugs listed at the US FDA, 62 are currently in short supply. Out of those 62 drugs, 48 medicines are exported from Indian ports. However, only 23 of the short supply drugs are exported for exclusive population of the US. The remaining 25 medicines are exported to European, African, Middle East and nearby Asian countries where the demand is still in the nascent phases.Most of the medicines which are in short supply are injectables formulations particularly antibiotics (mainly cephalosporins). The results are then compiled to ascertain the possible potential of Indian drug exports of these products. Research shows that 19 key drug products are exported from India where USA is the largest consumer. Needs to be seen that for remaining drugs in the total of 62 short supply drugs, there stands a tremendous potential for the Indian pharmaceutical companies to enhance their export potential.
The future
The Indian pharmaceutical industry has dual avenues to enhance its export potential. One is the path of the regular ANDA approvals which despite being a slow and lengthy processes at the US FDA continues to remain the sunrise for the Indian pharmaceutical industry.
The second path is the short supply drug market which is one of the most important path for the growth of the Indian pharmaceutical exports. The US FDA website lists around more than 100 drugs, mainly injectables, which are in short supply. The issue of short supply for around 62 medicines is yet to be resolved by the USFDA. Of those 48 medicines are manufactured in India. But only around 19-20 medicines of those manufactured in India are exclusively shipped to USA.
Majority of the demand lies with the US market which accounts for more than 50 percent of the total export value in the short supply drug market. Despite the fact that more than 50 percent of the key shipments at the key ports are for the US consumption, there is still a short supply of drugs. This indicates that there exists a huge potential to simultaneously manufacture and export more drugs which are in the short supply in the US market.
___________________________________________________________________________________________________
Rashmi Pant is an expert in market research with more than 15 years of experience in major industrial sectors. She is also the owner of HOW TO: http://www.rashmipant.com
)