In this podcast, we will talk about the Covid Vaccine scenario, in which we will discuss trial results, efficacy among other things
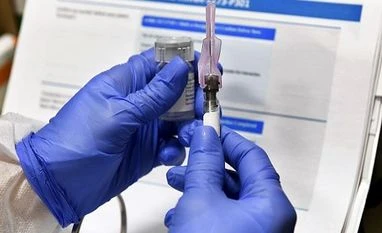
A senior government official informed on Monday that India is considering reviewing Pfizer Inc and AstraZeneca’s vaccines, produced by Serum Institute in India, for emergency use on an accelerated basis.
But, these two aren’t the only one in the queue as Hyderabad-based Bharat Biotech has also applied for an emergency use authorisation for its investigational vaccine candidate, Covaxin, to the Indian regulator. The firm is conducting its Phase 3 trial, which involves 26,000 participants.
Tune in for more
)