Leading domestic drug makers Lupin, Unichem and Matrix Labs (now part of Mylan) have come under the scanner of the European Commission for curbing entry of the low-cost generic version of cardiovascular medicine Perindopril in the European Union (EU).
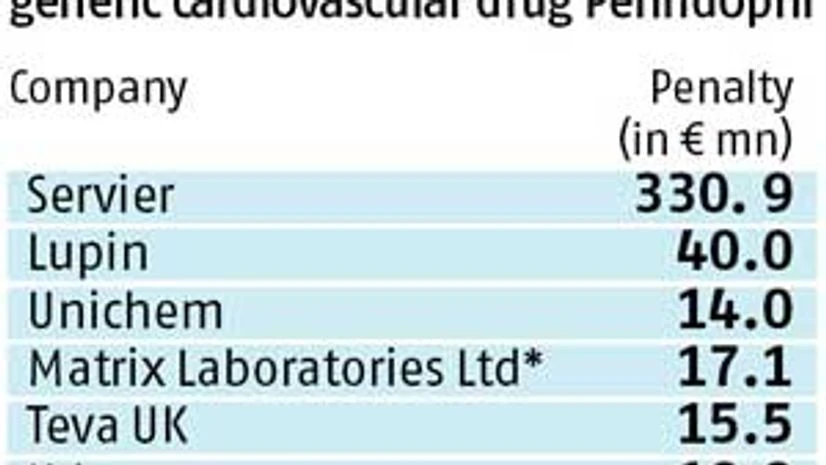
The anti-trust wing of the Commission has slapped a hefty fine of euro 427.7 million (around Rs 3,458.7 crore) on five generic drug manufacturers, besides Servier, a French pharmaceutical company that held patent protection for blockbuster Perindopril.
Of the total fine, Lupin has to pay euro 40 million (around Rs 324 crore), while Unichem and Matrix have been fined for euro 13.96 million (around Rs 113 crore) and euro 17 million (Rs 138 crore), respectively.
According to the Commission, Servier and the five generic drug producers concluded a series of deals — all aimed at protecting the innovator company’s bestselling blood-pressure medicine from price competition from generics in the European Union.
“Through a technology acquisition and a series of patent settlements with generic rivals, Servier implemented a strategy to exclude competitors and delay the entry of cheaper generic medicines to the detriment of public budgets and patients, in breach of the EU anti-trust rules,” the Commission said in a statement on Wednesday.
Drug maker Lupin had sold certain patent applications and other related intellectual property on Perindopril to the French company in 2007 for around euro 20 million.
“We are very disappointed with the European Commission’s findings. We are still awaiting an official copy of the decision. We remain confident of our position and intend to appeal against the decision,” a spokesperson for Lupin said.
Apart from the Indian drug makers, the European watchdog also penalised Israel’s Teva and Slovenia-based Krka for their alleged anti-competitive actions.
In 2003, most of Servier’s patents for the perindopril molecule expired, though some of the secondary patents related to processes and forms continued, the competition regulator said.

According to the regulator, at that time, generic drug makers of perindopril were “intensively preparing” their market entry and sought access to patent-free products or challenged the Servier patents blocking them. “There were very few sources of non-protected technology. In 2004, Servier acquired the most advanced one, forcing a number of generic projects to stop and, therefore, delaying their entry,” the Commission explained in the statement.
With their way to the market cut off, generic producers decided to challenge Servier’s patents before courts. However, between 2005 and 2007, virtually each time a generic company came close to entering the market, Servier and the company in question settled the challenge.
“This was not an ordinary transaction where two parties decided to settle a patent claim outside of court to save time and costs. Here, the generic companies agreed to abstain from competing in exchange for a share of Servier’s rent,” the regulator said.
Citing examples of such settlements, which according to the Commission happened at least five times between 2005 and 2007, the EU regulator said Servier offered a generic company a licence for seven national markets; in return, the generic drug maker agreed to “sacrifice” all other EU markets and stop efforts to launch its perindopril there.
“In total, cash payments from Servier to generics amounted to several tens of millions of euros,” the Commission said.
Commission Vice-President Joaquín Almunia, in charge of the competition policy, said: “Servier had a strategy to systematically buy out any competitive threats to make sure they stayed out of the market. Such behaviour is clearly anti-competitive and abusive. Competitors cannot agree to share markets or market rents instead of competing, even when these agreements are in the form of patent settlements. Such practices directly harm patients, national health systems and taxpayers.”
While the European watchdog has imposed a fine of euro 331 million on Servier, the remaining fine of about euro 100 million is split among the five producers of generic medicines on the basis of the extent of their involvement.

)
