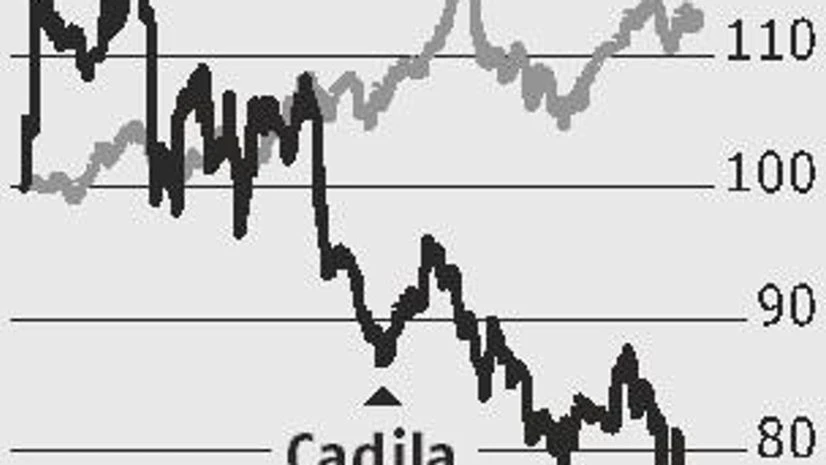
Cadila Healthcare, unlike many of its peers, seems to be a bright spot in the pharma space. The company, which has been buzzing with news on approvals of drug launches, announced on Wednesday the USFDA’s approval for its Ahmedabad-based SEZ facility. The unit, which manufactures oncology injectables for regulated markets, was inspected from 28 May to June 5 and has not received observations by the drug regulator.
With this, Cadila, which resolved the FDA warning letter for its Moriaya plant last year, has no regulatory overhang. In fact, the strong product pipeline for the US market and faster pace of approvals

)