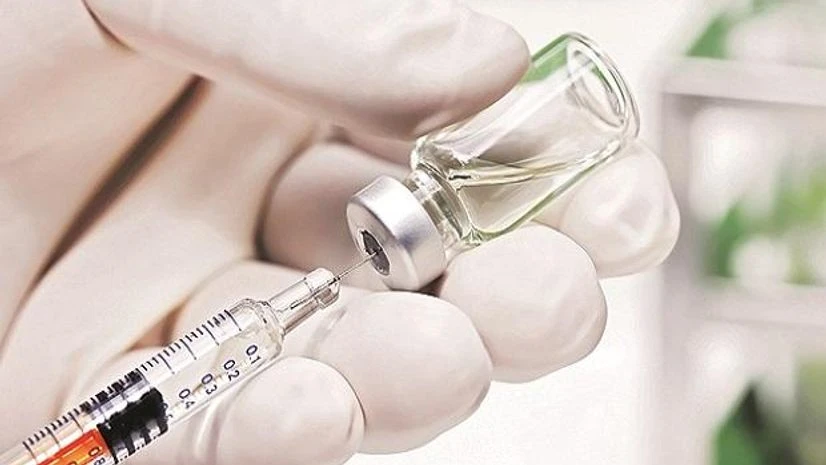
The US drug regulator has approved Biocon’s Semglee, the first interchangeable biosimilar insulin product, to treat diabetes patients. This means that Semglee can be substituted for its reference drug – Sanofi’s Lantus – by a pharmacist in the US without any intervention of the prescriber. Lantus is a long-acting insulin glargine.
The move will empower patients by “helping to increase access to safe, effective and high-quality medications at potentially lower cost”, the US Food and Drug Administration (USFDA) said in a statement. Semglee will be manufactured by Biocon Biologics, a subsidiary of the Bengaluru-headquartered firm, and marketed by its partner

)