India's drug regulator Drugs Controller General of India (DCGI) on Saturday approved an anti-coronavirus drug for emergency use to treat moderate and severe patients. It is developed by Defence Research and Development Organisation (DRDO) in collaboration with Dr Reddy’s Laboratories (DRL).
The drug comes in powder form in sachet, which is to be taken orally by dissolving it in water. It accumulates in the virus infected cells and prevents virus growth by stopping viral synthesis and energy production.
"The clinical trials of the drug, drug 2-deoxy-D-glucose (2-DG), have shown that this molecule helps in faster recovery of hospitalised patients and reduces supplemental

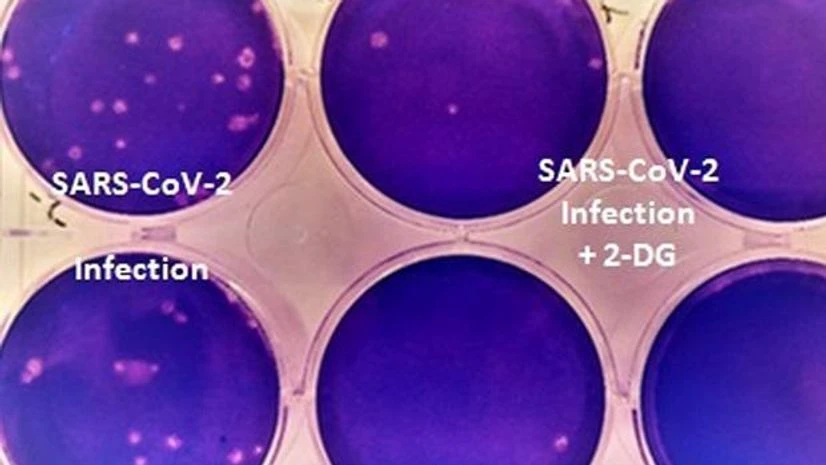)