The 2-DG drug developed by the
Defence Research and Development Organisation (DRDO) could be a game-changer in the fight against COVID, Karnataka Health Minister Dr K Sudhakar said on Friday.
"The 2-DG drug developed by DRDO is a big breakthrough and could be a game-changer in the battle against pandemic as it helps in faster recovery of hospitalised patients and reduces oxygen dependence," Sudhakar was quoted as saying in a statement issued by his office.
The Minister had visited the DRDO campus in the city where scientists briefed him about the ongoing efforts at the premier research organisation to find solutions to tackle the pandemic.
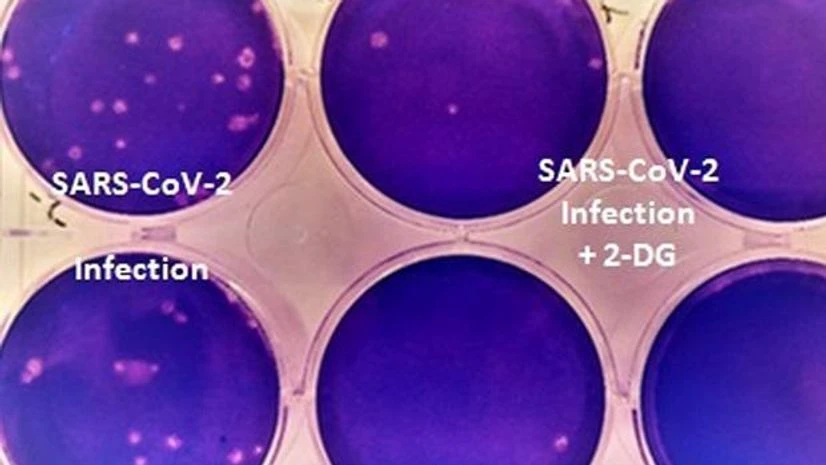
About the 2-DG (2-deoxy-D-glucose), an anti-COVID-19 therapeutic application of the drug, the statement read that it has been developed by the Institute of Nuclear Medicine and Allied Sciences (INMAS), a lab of Defence Research and Development Organisation (DRDO), in collaboration with Dr Reddys Laboratories (DRL), Hyderabad.
Clinical trial results have shown that this molecule helps in faster recovery of hospitalised patients and reduces supplemental oxygen dependence.
Higher proportion of patients treated with 2-DG showed RT-PCR negative conversion in COVID patients.
More From This Section
The drug would be of benefit to the people suffering from COVID-19, the statement read.
In April 2020, during the first wave of the pandemic, INMAS-DRDO scientists conducted laboratory experiments with the help of Centre for Cellular and Molecular Biology (CCMB), Hyderabad, and found that this molecule works effectively against SARS-CoV-2 virus and inhibits the viral growth, according to the statement.
It said based on these results, the Drugs Controller General of Indias (DCGI) Central Drugs Standard Control Organisation (CDSCO) permitted Phase-II clinical trial of 2- DG in COVID-19 patients in May 2020.
The DRDO, along with its industry partner DRL, Hyderabad, started the clinical trials to test the safety and efficacy of the drug in COVID-19 patients.
In Phase-II trials (including dose ranging) conducted during May-October 2020, the drug was found to be safe in COVID-19 patients and showed significant improvement in their recovery, the statement said adding, Phase-II was conducted in six hospitals and Phase IIb (dose ranging) clinical trial was conducted at 11 hospitals all over the country.
Phase-II trial was conducted on 110 patients.
Another innovative solution of DRDO - the Oxycare System - optimises the consumption of oxygen and reduces the workload and exposure of healthcare providers by eliminating the need of routine measurement and manual adjustments of oxygen flow, he added.
According to the Minister, the PM-CARES Fund would procure 1.5 lakh units of Oxycare System at a cost of Rs 322.5 crore.
(Only the headline and picture of this report may have been reworked by the Business Standard staff; the rest of the content is auto-generated from a syndicated feed.)

)
