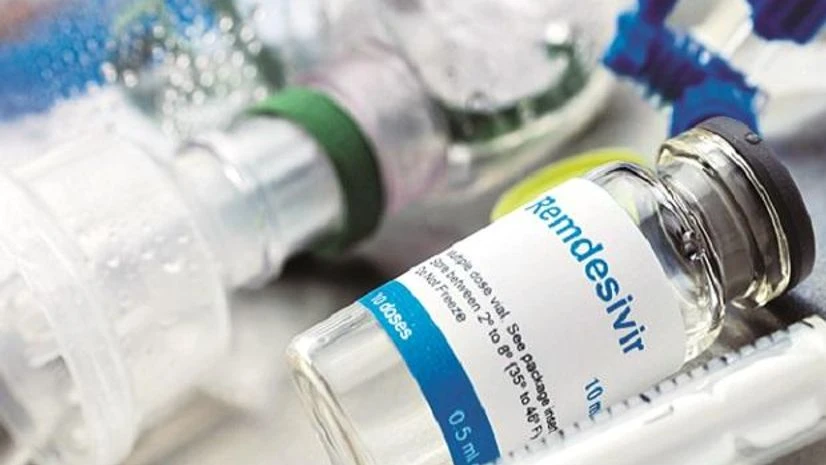
The Centre onTuesday said doctors should ensure "rational and judicious" use of anti-viraldrug Remdesivir, underlining it is to be given only to serious COVID-19 patients in hospitals and is not to be used in home settings.
At a weekly press conference, NITI Aayog member (Health) Dr V K Paul said, "Remdesivir is to be usedonly in those who require hospitalisation and are onoxygen support. That is the precondition. There isno question of its use in the home setting and for mild cases, and it is not to be procured from chemist shops."
Remdesivir is listed for use in serious COVID-19 patientsin the Clinical ManagementProtocols for COVID-19 as aninvestigational therapy.
As the shortage of Remdesivir was reported in some areas, itsexport was banned and the medicine is available in plenty, Paul said, adding that "queuing outside chemist shops to procure Remdesivir is creating distortions".
"We appeal to physicians to ensure rational, correct and judicious use of Remdesivir in hospitalised patients," he said.
In view of a sudden spike in demand due to the surge in COVID-19 cases,India on Sunday banned the export of injectionRemdesivirandRemdesivirActive Pharmaceutical Ingredients (API) till the situation improves.
Also Read
To ensure easy access of hospitals and patients toRemdesivir, alldomestic manufactures ofRemdesivirhave also been advised to display on their website, details of their stockists/distributors to facilitate access to the drug, the Union Health Ministry had said.
Drug inspectors and other officers have been directed to verify stocks and check their malpractices and also take other effective actions to curb hoarding and black marketing.
The State Health Secretaries will review this with the drug inspectors of the respective states and UTs, the ministry had said on Sunday.
The Centre has also advised the states that the extant 'National Clinical Management Protocol for COVID-19', which is based on evidence, has been developed after many interactions by a committee of experts and is the guiding document for the treatment of COVID-19 patients.
In the Protocol,Remdesiviris listed as an investigational therapy, i.e. where informed and shared decision-making is essential, besides taking note of contraindicationsmentioned in thedetailed guidelines, the ministry said.
The states and UTs have been advised that these steps should again be communicated to all hospitals, both in the public and private sector, and compliance monitored.
(Only the headline and picture of this report may have been reworked by the Business Standard staff; the rest of the content is auto-generated from a syndicated feed.)

)
